As experts in Organic Reaction Process Development (Molecular Synthesis) and Manufacturing, we specialize in synthesizing organic fine molecules using multi-step processes to create complex organic molecules, including active pharmaceutical ingredients (APIs), drug intermediates, and drug metabolites on scales ranging from milligrams to multi-kilograms.
Our custom synthesis services utilize key strategies such as process and product analysis to identify ingredients, solvents, and changes in temperature and pH during chemical reactions or transformations. To ensure successful reactions, we employ Reaction Progress Indicating Method (RPIM) and Product Stability Indication Method (PSIM) to monitor the reaction process and avoid over-reaction and further degradation and hydrolysis.

In the API Synthetic Processes which is mainly the Organic Reaction Process, it’s essential to monitor the composition of reaction mixtures containing reactants, intermediates, products, by-products, degraded, decomposed, and hydrolyzed products, as well as process-related impurities. We use chromatographic separation and identification/qualification and quantification techniques to identify, qualify, and quantify all the entities in a reaction mixture.
As part of the compound synthesis process, the resulting compound is purified using various techniques, such as extraction-evaporation, distillation, chromatographic separation, and crystallization. Once the desired quantity is obtained, the compound undergoes further processing to remove any remaining water and volatile organic solvents. This drying process is crucial for preserving and storing the compound. To achieve optimal drying, we use various methods such as Fluidized Bed Drying and Spray Drying. For heat-sensitive or labile molecules, we use a specialized technique called Lyophilisation (Freeze Drying). This process ensures that the compound is thoroughly dried while maintaining its stability to air, moisture, and heat.

We specialize in developing cost-effective monitoring processes for organic reaction processes using peak identification and qualitative determination of each entity through TLC-UV and TLC using different detection methods. We then optimize the reaction process by quantifying each entity to achieve maximum output or high yield using HPLC separating systems with different detection methods including UV/PDA/MS/MS. In addition, we develop analytical methods using HPLC-UV/PDA/MS/MS for quantifying individual molecular species. This is a critical and crucial process for both qualifying and quantifying the target molecule/compound with reference to reference standard compounds. We then develop a separation sequence, optimize retention, optimize efficiency, optimize separation, and achieve complete resolution, as shown in Fig-1. To optimize the separation method, we study the effects of various factors such as pH, stationary phase composition, mobile phase composition, nature of solvent (strength and polarity), solvent ratio, flow rate, temperature, and more.
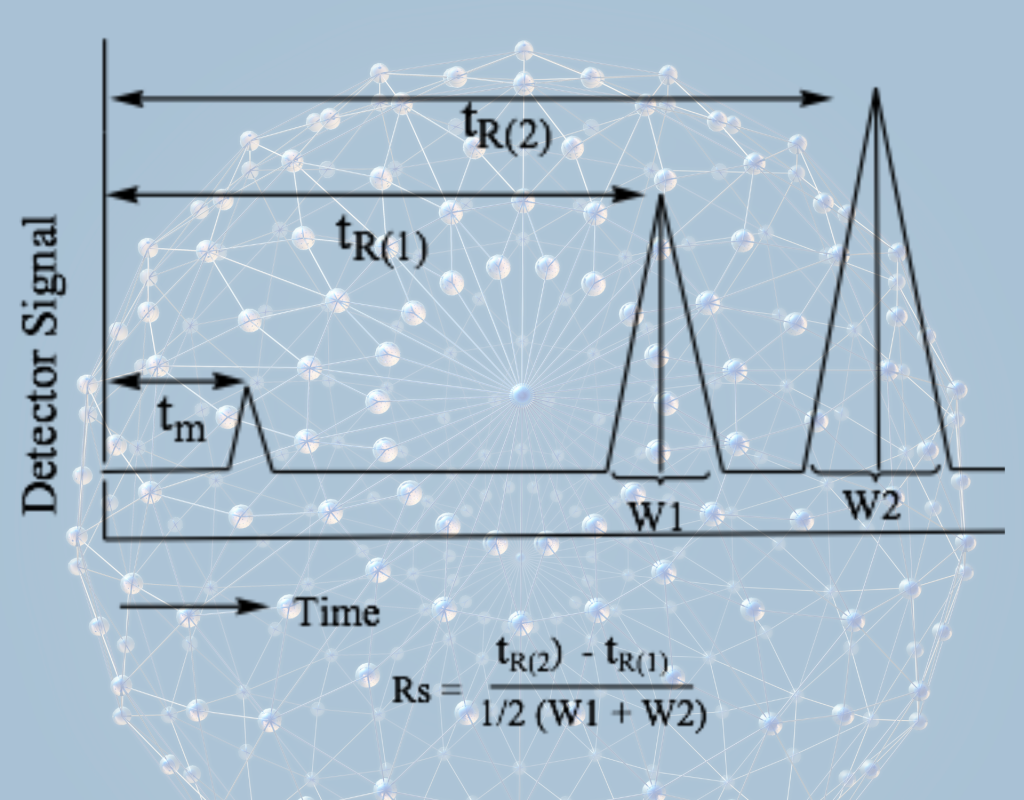
Important statistical data, the key parameters, be determined for validation of the method such as Selectivity/Specificity, Linearity, Precision, Robustness, Limit of Detection and Limit of Quantitation
Drug Substance and Related Impurities identification and quantification by GC-FID/TCD/MS/MS validated methods if the compound is not thermal labile. For thermally labile compound we utilize the HPLC-UV/PDA/MS/MS method.
We provide services for the required physiochemical characterization of API’s, the drug substances. When drug substance properties are well understood, problems in later stages of drug product development are minimized, drug product development costs are reduced and the time from development to market is decreased.
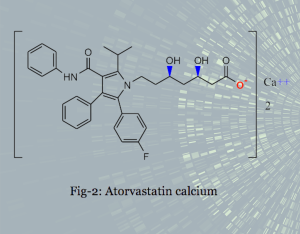
We provide generic drug substance manufacturing process development services such as Atorvastatin Calcium:




At our company, we understand the importance of developing and analyzing dosage formulations in the pharmaceutical drug product development process to ensure the final products meet the necessary standards of safety, efficacy, and quality. Our team of experts specializes in developing ingestible and topical products such as softgel capsules, liquid-filled hard shell gelatin and vegetable capsules, orally dissolving tablets, and transdermal drug products.
We offer pre-formulation and stable formulation development services for drug products, utilizing Design of Experiment (DOE) techniques to optimize and scale-up formulation processes. Our team also conducts formulation process validation and technology transfer to ensure a smooth transition to production.
We conduct quality analysis of drug products using liquid chromatographic methods to identify drug substance and degradation products arising from hydrolysis, thermal and oxidative decomposition. Our team conducts HPLC method development to separate the active ingredient from related substances and degraded products using appropriate columns, mobile phases, and detectors.
We also specialize in identifying and resolving quality issues related to softgel capsules, such as leaking and slow dissolution, which can lead to low bioavailability.
Our company offers services for the development of nutraceutical and phyto drug products, which are natural health products derived from marine and terrestrial plants and herbs that offer a range of therapeutic properties. We provide services to identify the right bioactive compounds in these products and develop dosage forms such as tablets, hard shell capsules, softgel capsules, creams, and liquids to deliver the best possible treatment for symptoms.
Our consultancy services cover all aspects of setting up and managing Organic Reaction Process facilities, from GLP lab set-up to GMP pilot plant facilities. Our team of experts will work with you to identify your specific needs and recommend the appropriate equipment for your Active Pharmaceutical Ingredients (API) manufacturing process.
We offer consultancy services for setting up GLP labs and GMP pilot plants to ensure compliance with regulatory requirements. Our team of experts will work with you to design and implement a facility that meets your needs and complies with all regulatory requirements.
Our Organic Reaction Process experts can help you identify if setting up a multi-product API manufacturing plant is feasible for your needs. This can help you manufacture multiple API products in one facility, which can lead to increased efficiency and cost savings.
Managing toxic and explosive chemicals is a crucial aspect of Organic Reaction Process facilities. Our team of experts provides technical consultancy and training services for safe handling, storage, and disposal of these chemicals. We ensure that all necessary precautions are taken to ensure the safety of your employees and the environment.
Share
Copyright © 2023 Molesci Matesci. All rights reserved.